Neuronetics Research
Neuronetics has developed the NeuroStar TMS Therapy® system, the first and only non-systemic and non-invasive depression treatment cleared by the FDA for patients who have not benefited from prior antidepressant treatment.* NeuroStar has a reliable safety record, with over 10,000 active treatments safely performed across all NeuroStar clinical trials.1 NeuroStar TMS therapy significantly improved patient scores on the Montgomery Asberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HAMD-24) scale.2 View the research.
Through 23 premier research institutions internationally, Neuronetics completed the largest clinical trial ever conducted evaluating efficacy and safety of TMS Therapy in the treatment of major depression.
Clinical Trials Sites:
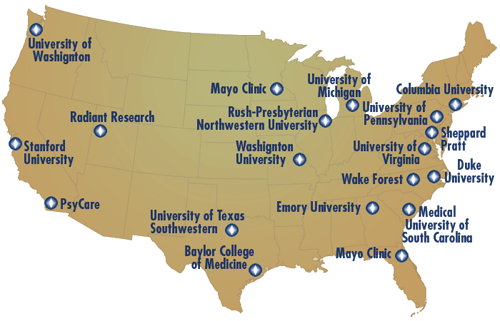
Three Premier International Centers:
1. Centre for Addiction and Mental Health, Toronto, Canada
2. Black Dog Institute, Sydney, Australia
3. Alfred Psychiatry Research Centre, Melbourne, Australia
Please note: The NeuroStar TMS Therapy trials have been completed and are no longer enrolling patients. If you or a loved one is suffering from major depression, you can find a NeuroStar TMS Therapy provider in your area. Find a provider.
References:
Janicak, P, et al. Transcranial Magnetic Stimulation (TMS) in the Treatment of Major Depression: A Comprehensive Summary of Safety Experience from Acute Exposure, Extended Exposure and During Reintroduction Treatment. Journal of Clinical Psychiatry, 2008; Vol 69:222-232.
Thase M, Demitrack M, Evaluating Clinical Significance of Treatment Outcomes in Studies of Resistant Major Depression, Biological Psychiatry, April 1, 2008; Vol. 63:7s Page, 138s.
* NeuroStar TMS Therapy® is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.